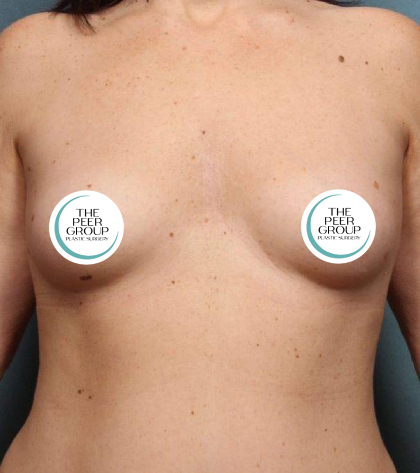
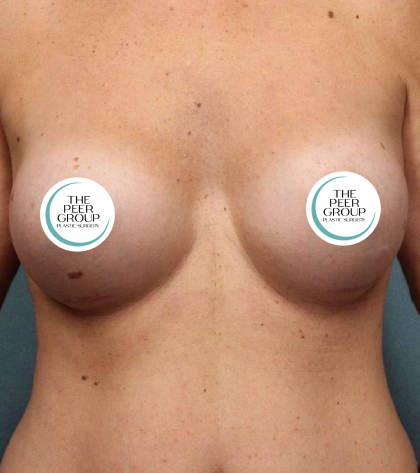
Breast Implant Information

Important Information For Breast Implants At The Peer Group New Jersey
In light of the announcement by Allergan regarding their TEXTURED breast implants and tissue expanders, the board-certified plastic surgeons of The Peer Group are encouraging all of our breast implant patients to contact our office if they have any concerns. We would like to emphasize that the recall is for textured Allergan implants that are on the shelf. There is NO recommendation from Allergan, or the FDA, that these implants and expanders need to be prophylactically removed if they have already been implanted.
Your doctor will be able to discuss with you the type of implant you have and answer your questions. We encourage all of our breast implant patients who have not been seen within the past year to make an appointment to be seen as part of their routine follow-up. As always, your health is our primary concern.
The board-certified plastic surgeons at The Peer Group remain committed to providing our patients with the most current and accurate information possible. In addition to the provided links below, we are always available to meet with you to discuss any concerns that you may have.
We look forward to assisting you.
More Information
- https://www.surgery.org/sites/default/files/PATIENT-FAQ-BIA-ALCL-FINAL-12-3-18.pdf
- https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-patients-risk-certain-textured-breast-implants-requests-allergan
- https://www.allergan.com/biocellresources
- BIA-ALCL Information from the American Society of Plastic Surgeons
- FDA letter 5/3/2019 Summarizing recent FDA committee findings and recommendations
- FDA Reports of Breast Implant Associated Anaplastic Large Cell Lymphoma