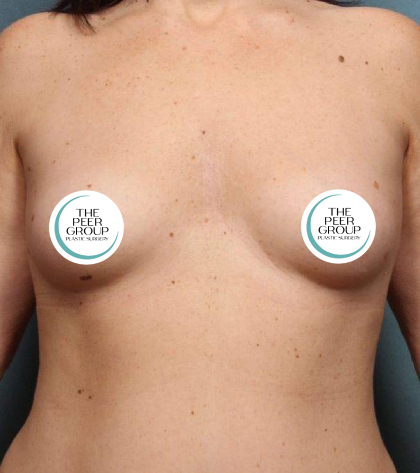
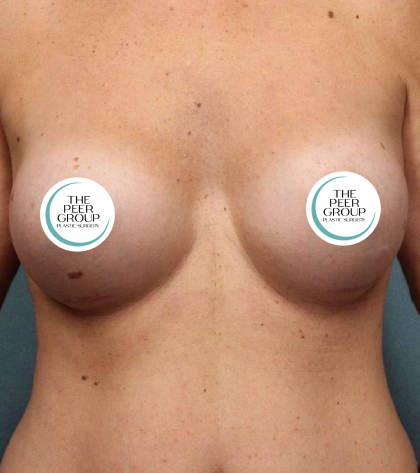
In light of the recent announcement by Allergan regarding their TEXTURED breast implants and tissue expanders, the doctors of The Peer Group are encouraging all of our breast implant patients to contact our office if they have any concerns. We would like to emphasize that this current recall is for textured Allergan implants that are on the shelf. There is NO recommendation from Allergan, or the FDA, that these implants and expanders need to be prophylactically removed if they have already been implanted. Your doctor will be able to discuss with you the type of implant you have and answer your questions. We encourage all of our breast implant patients who have not been seen within the past year to make an appointment to be seen as part of their routine follow-up. As always, your health is our primary concern.
We look forward to assisting you.
Links associated with this topic:
- https://www.surgery.org/sites/default/files/PATIENT-FAQ-BIA-ALCL-FINAL-12-3-18.pdf
- https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-patients-risk-certain-textured-breast-implants-requests-allergan